FluidFM®: Fluidic force microscopy
The established microfluidic tool for nanomanipulation and single-cell biology
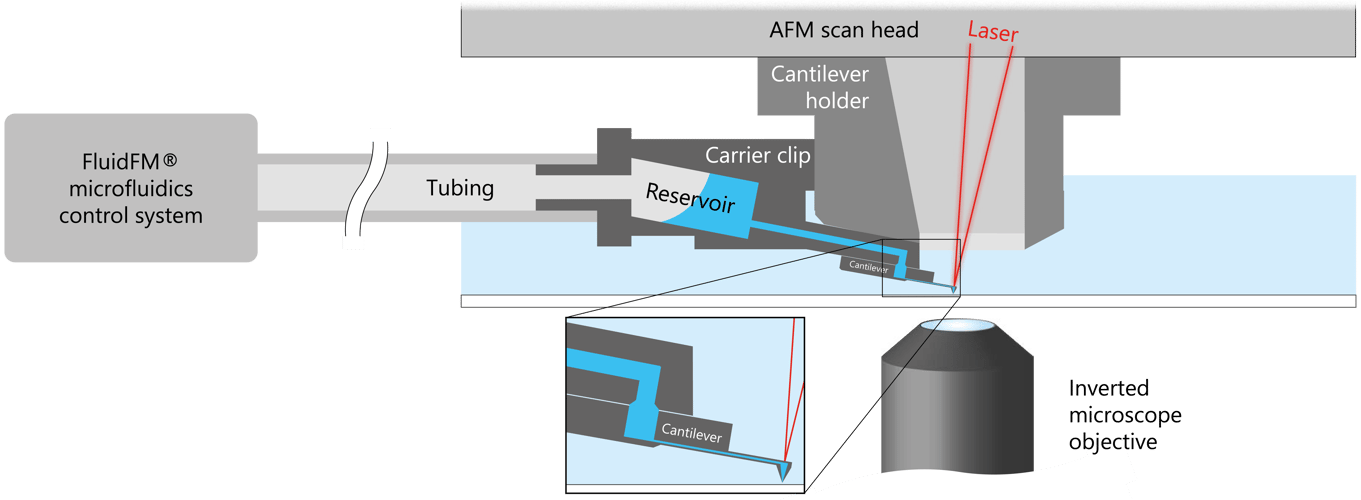
FluidFM literally brings microfluidics to the tip of an AFM cantilever. This way, it combines the microfluidics with the force sensitivity and positional accuracy of an AFM allowing a range of exciting applications in single-cell biology and nanoscience.
Nanosurf has a long experience providing AFM systems with FluidFM having launched the FlexAFM with FluidFM in 2011 together with Cytosurge AG. This integration of FluidFM on Nanosurf platforms has grown in time and now the FluidFM technology available for DriveAFM, FlexAFM, and CoreAFM platforms.

How does FluidFM work?
At the heart of the technology lies a hollow cantilever with an aperture at the tip, through which fluids are delivered or withdrawn. Liquid is filled into a small reservoir attached to the cantilever. Air pressure is applied to the other side of the reservoir through tubing, propelling the liquid through the channel in the cantilever and out of the aperture at the tip. By controlling the pressure and the flow rate of the liquid, FluidFM can be used to perform a wide range of tasks, including printing, dispensing, and probing.
The AFM component of FluidFM allows for precise positioning and force measurements at the nanoscale level. The microfluidic component allows for precise control of the amount and location of the liquid being delivered or withdrawn.
The combination of these capabilities allow for the manipulation of individual cells, bacteria, or other biological structures, as well as to deposit and remove materials with high accuracy, making it a powerful tool for a variety of applications in life sciences, materials science, and nanotechnology.
FluidFM application areas
With FluidFM you can perform a wide range of experiments
Different applications require different FluidFM probes
Different applications require different FluidFM probes
- FluidFM micropipettes: tipless cantilevers with opening at the cantilever end
- FluidFM nanopipettes: cantilevers with opening at the tip apex
- FluidFM nanosyringes: cantilevers with opening at the side of the tip, maintaining a sharp apex to penetrate biological membranes for injection and extraction
Highly accurate pressure, force, and position control with optical sample access
- From add-on to fully integrated system in the DriveAFM Studio software
- Fast and accurate FluidFM microfluidics control system
- Integrates with optical microscopy techniques, e.g. fluorescence
- Highest force resolution and up to 20 µm z-range internally
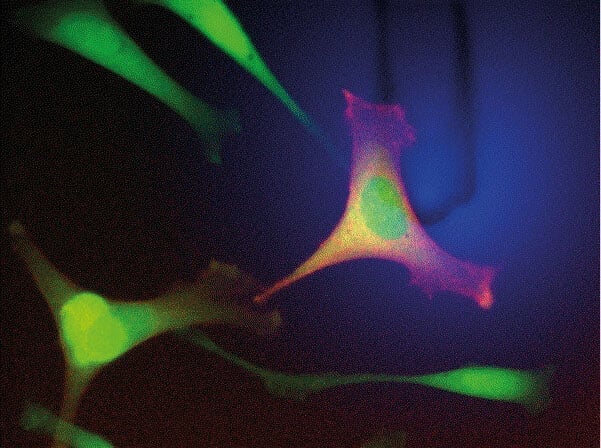

Optical cell selection by fluorescence properties (left image). The cantilever was gently placed on the cell under force control and trypsin administration was monitored from a co-deposited fluorescence dye (blue). After release, the cell was picked up with the same cantilever and isolated from the rest by placing it in a separate well, sorted by fluorescence (right image; adapted from Lab Chip (2014) 14, 402‑414 with permission from The Royal Society of Chemistry). Data courtesy: O. Guilaume-Gentil, ETH Zürich, Switzerland.
A tool for research pioneers
- FluidFM enables original research at the frontiers of science from bacterial adhesion to cell-cell interaction and from spotting to injection and extraction
- New: FluidFM can be combined with PicoBalance to measure the mass of cells and microparticles
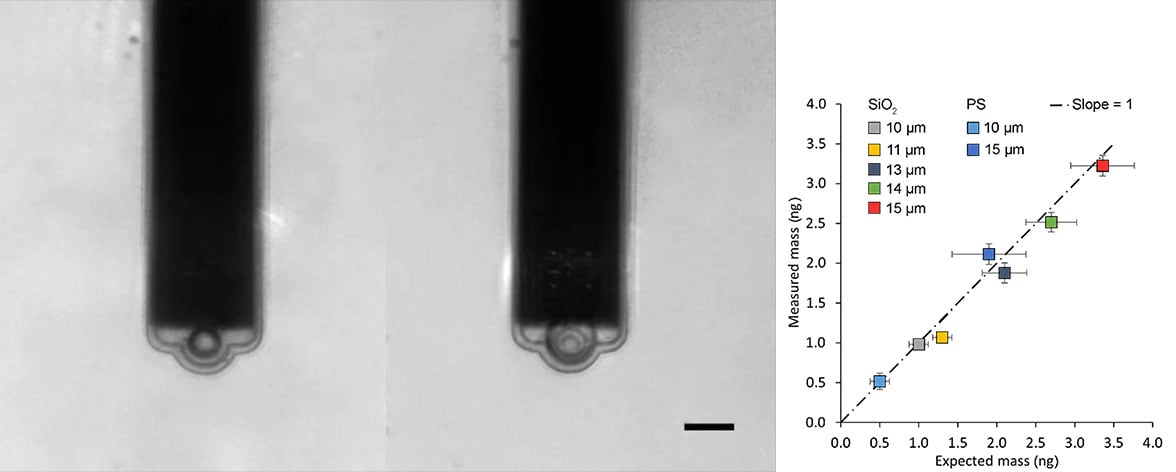
Left: Optical microscope image of FluidFM micropipettes with differently sized colloidal particles attached.
Right: PicoBalance mass measurement results for 7 different beads.
An integrated solution with optical, force, and fluidic control

FluidFM probes
All FluidFM probes come in sterile blister packs and pre-mounted on plastic carrier clips. Each blister pack has a QR code that can be read with the QR code reader supplied with the FluidFM system.
Cantilevers are easily mountable - even when wearing gloves.
Basic integration with FlexAFM and CoreAFM
FluidFM can be operated with basic integration for FlexAFM and CoreAFM, providing microfluidics control during the contact phase in spectroscopy for adhesion, spotting, injection or extraction experiments.
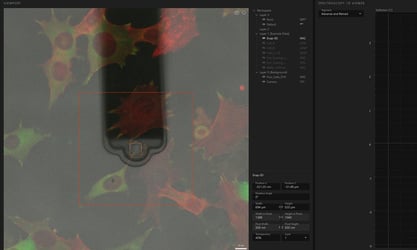
Perfect integration with DriveAFM and Nanosurf Studio
In the Studio software platform, FluidFM is seamlessly integrated. Besides access to the pressure controller during spectroscopy, Viewport allows targeting of cells or areas of interest directly in live feed of the optical camera. Furthermore DriveAFM uniquely provides the PicoBalance functionality: measure the mass of microparticles or single cells.

Prof. Tomaso Zambelli, Laboratory of Biosensors and Bioelectronics, ETH Zurich
FluidFM at ETH Zurich
At the Laboratory of Biosensors and Bioelectronics (ETH Zurich) we have been using the FlexAFM system with FluidFM add-on since the very early days of FluidFM. Together with Cytosurge, SmartTip and Nanosurf we developed this technology establishing several applications from cell adhesion, colloidal spectroscopy, force-controlled SICM and patch clamp, to local chemical cell stimulation. On the other hand, together with the Institute of Microbiology (ETH Zurich, Prof. Vorholt) we pioneered single-cell protocols like injection, extraction and isolation. Our results are well-received by the scientific community because of their originality stimulating ideas for experiments never attempted before. In this groundbreaking phase the Nanosurf team constantly supported us with interest, competence, and flexibility relying on their long-dated AFM experience.
FluidFM in action
Single cell injection of DAPI into the cytoplasm between two living Fibroblast cells. Performed with FluidFM integration on DriveAFM.
Example of single cell force spectroscopy: in this video a single cell is detached from fully adherent and confluent culture. The measured forces both depend on the substrate as well as the bonds to the neighboring cells. Courtesy of A. Sancho and J. Groll, Functional Materials for Medicine and Dentistry, University Hospital of Würzburg.
Working principle of single cell injection explained: Single cell nano-injection enables targeted introduction of foreign materials into a specific cell. Stimulating selected cells within a colony with your desired drugs or gene vectors provides new insights for life science, biology and medicine.
Example of nanolithography by FluidFM spotting. Droplets of paraffin oil are deposited on a glass substrate with a FluidFM nanopipette. Distance between spots down to approx. 5 µm in this spiral.
FluidFM is available on Nanosurf research AFM systems
FluidFM is available on Nanosurf's DriveAFM, FlexAFM and CoreAFM systems. Contact us to identify the right setup for your research requirements.

DriveAFM
The high-end solution for FluidFM

FlexAFM
The most published FluidFM solution

CoreAFM
The most affordable FluidFM solution
Download the FluidFM brochure

Related Resources
#{ item.resourceType }
#{ item.date_text_field }
#{ item.name }
#{ truncateText(item.metadescription) }
#{ item.readmoretext }